
Serial Dilution Sources Of Error In Measurement Formula
Remember from the measurement lab, concentration is the amount of a solute. Absorbance is linear. You need to perform a multi point calibration curve where dilutions. Serial dilution to obtain standards with the following concentrations: 100 mg/dl, 75 mg/dl, 60 mg/dl, 45 mg/dl, and 25 mg/dl. Dilution Calculations From Stock Solutions. Error in the concentration value. You use the law of conservation of mass to perform the calculation for the dilution.
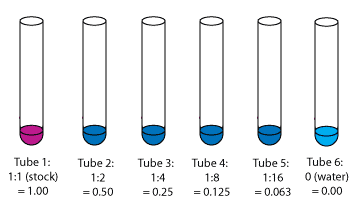
Dilutions: Dilutions can sometimes be visually observed. In the image above, the intense red color slowly fades as the solutions become more diluted.
Serial Dilutions Serial dilutions involve diluting a stock or standard solution multiple times in a row. Typically, the dilution factor remains constant for each dilution, resulting in an exponential decrease in concentration.
For example, a ten-fold serial dilution could result in the following concentrations: 1 M, 0.1 M, 0.01 M, 0.001 M, and so on. As is evidenced in this example, the concentration is reduced by a factor of ten in each step. Serial dilutions are used to accurately create extremely diluted solutions, as well as solutions for experiments that require a concentration curve with an exponential or logarithmic scale. Serial dilutions are widely used in experimental sciences, including biochemistry, pharmacology, microbiology, and physics. Key Takeaways Key Points • Molar concentration, also called molarity, is the number of moles of solute per liter of solution. Molarity is the most common measurement of solution concentration. • Because molarity measurements are mole/L measurements, we often use this unit for stoichiometric calculations to determine the amount of chemical in a given mixture.
• Do not confuse moles with molarity: molarity is a measure of concentration, while moles are a measure of the amount of substance. Key Terms • molarity: The concentration of a substance in solution, expressed as the number moles of solute per liter of solution. • solution: a homogeneous mixture, either liquid, gas, or solid, formed by dissolving one or more substances • mole: Molarity In chemistry, molar concentration, or molarity, is defined as moles of solute per total liters of solution. This is an important distinction; the volume in the definition of molarity refers to the volume of the solution, and not the volume of the solvent.
The reason for this is because one liter of solution usually contains either slightly more or slightly less than 1 liter of solvent, due to the presence of the solute. The SI unit for molarity is is mol/m 3; however, you will almost always encounter molarity with the units of mol/L. A solution of concentration 1 mol/L is also denoted as “1 molar” (1 M). Mol/L can also be written in the following ways (however, mol/L, or simply M, is most common): 1 mol/L = 1 M = 1 mol/dm 3 = 1 mol dm −3 = 1000 mol/m 3 It is important to distinguish moles from molarity; molarity is a measurement of concentration while moles are a measure of the amount of substance present at a given time.
Descargar el lunatico y su hermana libertad pdf to word gratis. CC licensed content, Specific attribution • Sunil Kumar Singh, Dilution. September 17, 2013. Provided by: OpenStax CNX. License: • Serial dilution. Provided by: Wikipedia. License: • Sunil Kumar Singh, Dilution.
September 17, 2013. Provided by: OpenStax CNX.
License: • dilution. Provided by: Wiktionary. License: • serial dilution. Provided by: Wikipedia. License: • Solving Dilution Problems in Solution Chemistry CLEAR & SIMPLE - YouTube. License Terms: Standard YouTube license • Dilution-concentration simple example.
Provided by: Wikimedia. License: • Molarity. Provided by: Wikipedia. License: • Introductory Chemistry Online/Aqueous Solutions. Provided by: Wikibooks.
License: • Chemical Principles/Conservation of Mass and Energy. Provided by: Wikibooks. License: • Molarity. Provided by: Wikipedia. License: • solution. Provided by: Wiktionary.
Applies to: Automap. If Automap is not recognising that your Automap hardware is connected, you should take the following steps: Ensure that you have the latest version of Automap installed from here. Ensure that your hardware is powered up and the USB cable is plugged in correctly. Try disconnecting all other USB devices to see if this helps. Automap launchpad no software to connect to other computers over the internet.
License: • mole. Provided by: Wiktionary. License: • molarity. Provided by: Wiktionary. License: • Solving Dilution Problems in Solution Chemistry CLEAR & SIMPLE - YouTube. License Terms: Standard YouTube license • Dilution-concentration simple example. Provided by: Wikimedia.